Research Projects
Prevention, diagnosis and treatment of antibody-mediated rejection
Antibody-mediated rejection (AMR) is a leading cause of kidney transplant failure. Our primary objective is the development of innovative diagnostic tools and treatment modalities to combat transplant injury triggered by antibodies.
There exists a significant unmet need for effective treatment strategies tailored to AMR. Furthermore, establishing timely and reliable methods for predicting humoral injury remains an ongoing challenge. Our current research focuses on evaluating novel treatment approaches to counteract antibody-mediated rejection processes. Over the past two decades, we have conducted several systematic prospective trials, including randomized controlled trials. Our first interventional trial, the AKARIS trial, an investigator-initiated open-label study designed to assess the efficacy of immunoadsorption in treating severe C4d-positive AMR, was prematurely terminated due to slow recruitment and a high graft loss rate in the control arm. Despite its small sample size, however, the results of this trial suggested considerable efficacy of immunoadsorption as an anti-humoral treatment strategy. The BORTEJECT study, sponsored by the Austrian Science Fund, was a randomized, placebo-controlled, double-blind phase 2 trial assessing the impact of the proteasome inhibitor bortezomib (administered in two cycles) versus placebo on transplant outcomes in patients with late AMR. Although the study did not show any significant effect of bortezomib on morphologic and molecular biopsy results or eGFR trajectories over 24 months, it provided valuable insights into various aspects of chronic AMR. Collaborating with Dr. Philip Halloran, director of the Alberta Transplant Applied Genomics Center 'ATAGC' in Edmonton, Alberta, Canada, we evaluated ABMR-related scoring of molecular signatures from biopsy materials using microarray-based technology. Additionally, in multidisciplinary projects analyzing the trial cohort, we have conducted a large number of in-depth side projects analyzing predictive donor-specific antibody characteristics, complement-associated biomarkers and genetics, NK cell functional genetics/immunohistochemistry, characteristics of microcirculation injury, and chemokines or donor-derived cell-free DNA as AMR-specific biomarkers. In collaboration with TrueNorth Therapeutics Inc. (South San Francisco, USA) and the Department of Clinical Pharmacology (Medical University of Vienna), we have investigated the effect of the anti-C1s monoclonal antibody TNT009 on HLA antibody-triggered classic pathway activation (Link). This project involved pre-clinical experimental studies and a phase 1 trial in healthy volunteers and in 10 patients with late AMR. The trial demonstrated profound classical complement inhibition, including a notable decrease in C4d staining in 1-month follow-up biopsies. However, short-term biopsy results argued against an effect of classical complement inhibition on inflammatory processes in the transplant microcirculation. In collaboration with the Department of Clinical Pharmacology (Medical University of Vienna), Berlin Charite Universitätsmedizin (Berlin, Germany), supported by Vitaeris, Inc. (South San Francisco, USA) and CSL Behring, we have subsequently conducted a randomized controlled phase 2 trial to assess the safety and tolerability of the anti-Interleukin-6 antibody clazakizumab in 20 recipients with late AMR. Clazakizumab was observed to decrease DSA levels and slightly modulate molecular AMR activity over 24 months, alongside a significant amelioration of eGFR loss over one year. However, the trial uncovered important safety signals, including two diverticular complications. Based on the results of this exploratory trial, a large multicenter phase 3 trial (IMAGINE) was designed (CSL Behring).Our current research endeavors focus on a new treatment paradigm targeting CD38 to interfere with NK and plasma cells. In an investigator-initiated randomized controlled bi-center phase 2 trial (Vienna, Berlin), supported by MorphoSys AG (Planegg, Germany) and Hi-Bio, Inc. (South San Francisco, CA, USA), we evaluated the safety and efficacy of the CD38 antibody felzartamab in 22 patients with late AMR. Showing acceptable safety and promising efficacy, including the resolution of morphologic and molecular AMR activity, depletion of circulating NK cells, and a marked reduction in dd-cfDNA, our trial results have now prompted the planning of a large international phase 3 trial (Hi-Bio, Inc.). The trial has now been extended (continuation of treatment in eligible patients), including a variety of ongoing research projects aimed at better understanding the mode of action of targeting CD38.
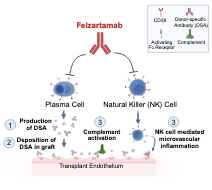
Targeting CD38 in antibody-mediated rejection
A current focus of our research is the concept of targeting CD38 in patients with antibody-mediated rejection. CD38 is a surface molecule highly extpressed on NK and plasma cells, and could act via a dual mode of action, depletion of NK effector cells and alloantibody-producing plasma cells. We have recently conducted a phase 2 trial to evaluate the concept in patients with late active or chronic active AMR.
This trial now revealed a beneficial safety profile and marked impact on a set of exploratory secondary efficacy endpoints. Notably, we observed a marked reduction of microvascular inflammation, associated with a significant modulation of AMR related transcript patterns. Interestingly, while dd-cfDNA was reduced there was no apparent impact on the levels of donor-specific antibodies. A marked reduction of peripheral NK cell counts, suggests that the main mode of action could be the depletion of NK effector cells. (Mayer KA, Schrezenmeier E, Diebold M, et al. A Randomized Phase 2 Trial of Felzartamab in Antibody-Mediated Rejection. N Engl J Med. Published online May 25, 2024. doi:10.1056/NEJMoa2400763)
The results of this trial now provide the basis for a future phase 3 trial, which is currently in the planning phase. The project is performed in a tight collaboration with Human Immunology Biosciences, Inc. (HI-Bio™). Based on the promising results, HI-Bio has received FDA Orphan Drug Designation for felzartamab for the treatment of AMR in kidney transplant recipients.
The VIETAC lab now focuses on performing studies to better understand the mode of action of felzartamab. Our analysis include a detailed evaluation of the molecular changes in study biopsies (collaboration with Phil Halloran, University of Alberta, Edmonton), a detailed evaluation of rejection biomarkers and characterisitics of donor-specific antibodies, spatial analysis using transcriptomics and multiplexed immunflorescene, as well as in vitro experiments to dissect the effect of felzartamab on NK cell activation.
Currently, the phase 2 trial has entered a trial extension to analyze the impact of prolonged treatment with felzartamab in study patients with persistent AMR activity.
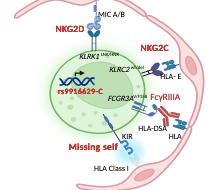
The role of NK cells in transplant rejection
There is increasing evidence of an important role for NK cells in organ transplantation.
NK cells can be activated by a variety of different receptors and inhibitory versus activating signals. Which factors modulate the activation of NK cells is not yet fully understood. This project investigates the role of NK genetics in kidney transplant rejection and elucidate its impact on allograft survival. We aim to identify factors that modulate the activation of NK cells and will lead to microvascular inflammation and long-term graft loss. It will further expand our view on the pathophysiology of kidney transplant rejection and pave the way for personalized approaches of risk stratification, facilitate the development of targeted therapies and better clinical outcomes
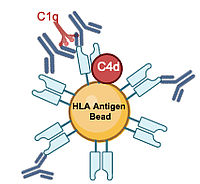
Desensitization and crossmatch-conversion of sensitized recipients of a deceased donor kidney allograft
Donor specific HLA antibodies (DSA) prior to transplantation are associated with positive complement dependent cytotoxicity crossmatch (CDCXM), antibody-mediated rejection (ABMR) and inferior allograft survival. Strategies to overcome immunological barriers are a major interest of our study group.
We particularly concentrate on developing desensitization strategies for HLA antibody-incompatible deceased donor transplantation. Since 2009, our unit has implemented prospective solid-phase monitoring for preformed DSA, with sensitized patients subjected to a peri-transplant initiation of immunoadsorption (IA) protocol, which was established in the 1990s. In a recent study, we analyzed a cohort of 101 recipients with DSA and/or crossmatch positivity who underwent peritransplant IA. Based on the study results we refined treatment allocation by evaluating intermediate-term transplant outcomes in relation to a comprehensive pre-transplant risk assessment, which included detailed solid phase antibody testing for DSA characterization and an assessment of various clinical and immunological variables (Link).

Torque Teno Virus for Immunologic monitoring after kidney transplantation
The TT virus is unique: it is naturally occurring in the blood of almost every healthy person and kidney transplant recipient but it causes no disease. If the immune system is strong, the TT virus load is low; this indicates a risk for organ rejection. If the immune system is weak the TT virus load is high; this indicates a risk for infection.
We believe that, the quantification of the TT virus load in the blood of kidney transplant recipients can help optimize immunosuppressive drugs and thus reduce infections and rejection. Within an EU funded project TT virus guided dosing of immunosuppressive drugs will be tested in a clinical trial including hundreds of kidney transplant recipients from all over Europe. For further information please visit the project webpage: ttv-guide.eu